Learning Outcomes
By the end of this lesson, students should be able to:
i. Define isomerism and its different types.
ii. Identify and differentiate between chain isomerism and positional isomerism in carboxylic acids.
iii. Classify carboxylic acids based on their isomeric forms.
iv. Appreciate the significance of isomerism in understanding the properties and reactivity of organic compounds.
Introduction
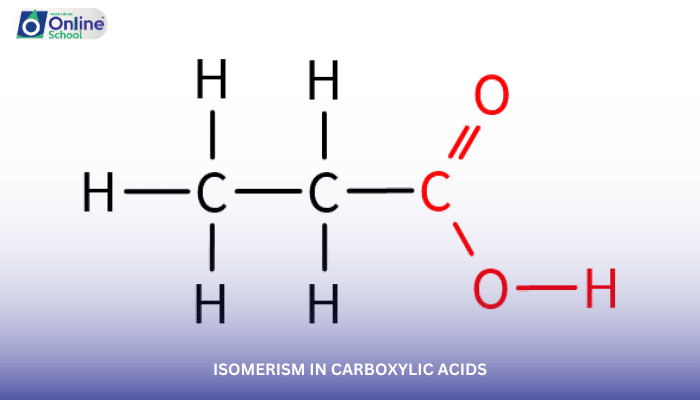
Isomerism, a fascinating aspect of organic chemistry, refers to the existence of compounds with the same molecular formula but different structural arrangements. Carboxylic acids, with their diverse structures, also exhibit isomerism, adding complexity and intrigue to their chemistry.
i. Chain Isomerism in Carboxylic Acids
Chain isomerism arises when the carbon atoms in the molecular chain are arranged differently, leading to compounds with distinct physical and chemical properties. In carboxylic acids, chain isomerism occurs when the carboxyl group (-COOH) is located at different positions along the carbon chain.
ii. Positional Isomerism in Carboxylic Acids
Positional isomerism, on the other hand, involves the attachment of substituent groups to the carbon chain at different positions. This type of isomerism is particularly relevant for carboxylic acids with substituents, as the position of the substituent can significantly impact the compound's properties.
iii. Identifying and Classifying Carboxylic Acid Isomers
To effectively identify and classify carboxylic acid isomers, it is crucial to carefully examine the arrangement of the carbon chain and the location of the carboxyl group, along with any substituents present. Chain isomers are distinguished by the length and branching of their carbon chains, while positional isomers differ in the placement of substituents.
Isomerism plays a pivotal role in understanding the diverse properties and behaviors of organic compounds. The existence of isomers with identical molecular formulas but different structures highlights the importance of molecular architecture in determining physical and chemical characteristics. This knowledge is essential for chemists in various fields, from designing new pharmaceuticals to understanding the intricate mechanisms of biological processes.
The study of isomerism in carboxylic acids provides a deeper understanding of these versatile organic compounds. By recognizing and differentiating between chain and positional isomers, students can appreciate the intricacies of molecular structure and its profound influence on the properties and reactivity of organic compounds. This knowledge forms the foundation for further exploration of organic chemistry and its diverse applications.